About clinical research
Clinical research comprises experiments or observations that are designed to answer specific questions affecting human health. There are various types of clinical research that are conducted at Karolinska Institutet. Clinical research takes shape in various forms. Some of the various types of clinical research conducted at KI are described accordingly.
What is clinical research?
Clinical research is a branch of healthcare science that evaluates medications, devices, diagnostic products, treatment regimens, behavioral interventions, preventive interventions and other efforts to improve human health. These may be used for prevention, treatment, diagnosis or for relieving symptoms of a disease. Different types of clinical research are conducted depending on the specific study objectives.
Types of clinical research
Below are descriptions of some of the different types of clinical research conducted at Karolinska Institutet.
- Treatment Research generally involves an intervention such as medication, psychotherapy, new devices, or new approaches to surgery or radiation therapy.
- Prevention Research looks for better ways to prevent disorders from developing or returning. Different kinds of prevention research may study medicines, vitamins, vaccines, minerals, or lifestyle changes.
- Diagnostic Research refers to the practice of looking for better ways to identify a particular disorder or condition.
- Screening Research aims to find the best ways to detect certain disorders or health conditions.
- Quality of Life Research explores ways to improve comfort and the quality of life for individuals with a chronic illness.
- Genetic studies aim to improve the prediction of disorders by identifying and understanding how genes and illnesses may be related. Research in this area may explore ways in which a person’s genes make him or her more or less likely to develop a disorder. This may lead to development of tailor-made treatments based on a patient’s genetic make-up.
- Epidemiological studies seek to identify the patterns, risks, causes, and control of disorders in groups of people.
Phases of clinical trials
Clinical trials are a type of clinical research designed to evaluate and test new interventions such as new investigational medications or medical devices. Clinical trials are often conducted in four phases. The trials at each phase have a different purpose to help answer specific questions at different periods during the clinical trial process.
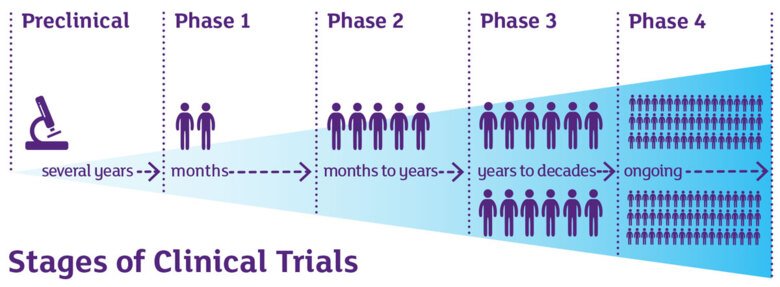
Phase I trials
- Researchers test an experimental drug or treatment in a small group of people for the first time. The researchers evaluate the treatment’s safety, determine a safe dosage range, and identify side effects.
Phase II trials
- The experimental drug or treatment is given to a larger group of people to see if it is effective and to further evaluate its safety.
Phase III trials
- The experimental study drug or treatment is given to large groups of people. Researchers confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the experimental drug or treatment to be used safely.
Phase IV trials
- Post-marketing studies, which are conducted after a treatment is approved for use by the EMA or FDA, provide additional information including the treatment or drug’s risks, benefits, and best use.
