Overview of the registration and reporting process
The process for registering and reporting results for clinical trials and clinical studies at Karolinska Institutet (KI) are based on specific regulations and requirements stipulated by national and international institutions, ethical obligations, and clinical trial registries. The most common trial registry for registering clinical studies is ClinicalTrials.gov, while all clinical trials are registered in CTIS.
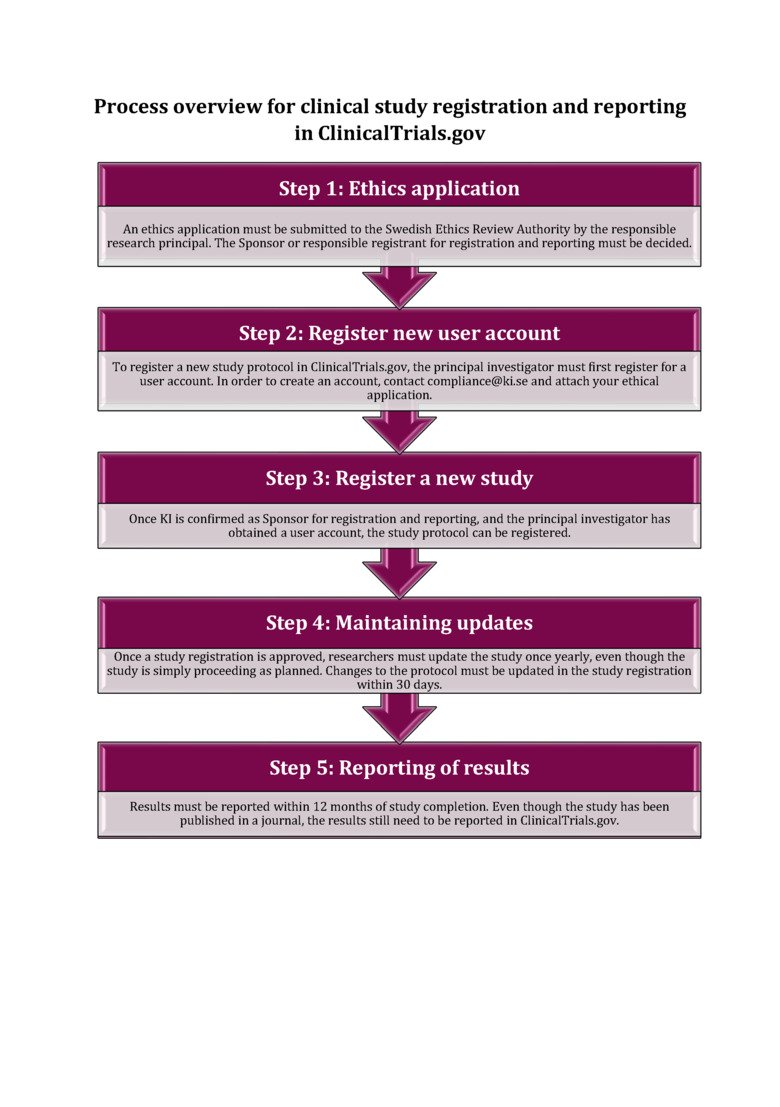
ClinicalTrials.gov
Below you will find a flow chart with a brief description of the different steps of registering a clinical study in ClinicalTrials.gov. For more detailed information on each step, please visit our webpage on ClinicalTrials.gov.
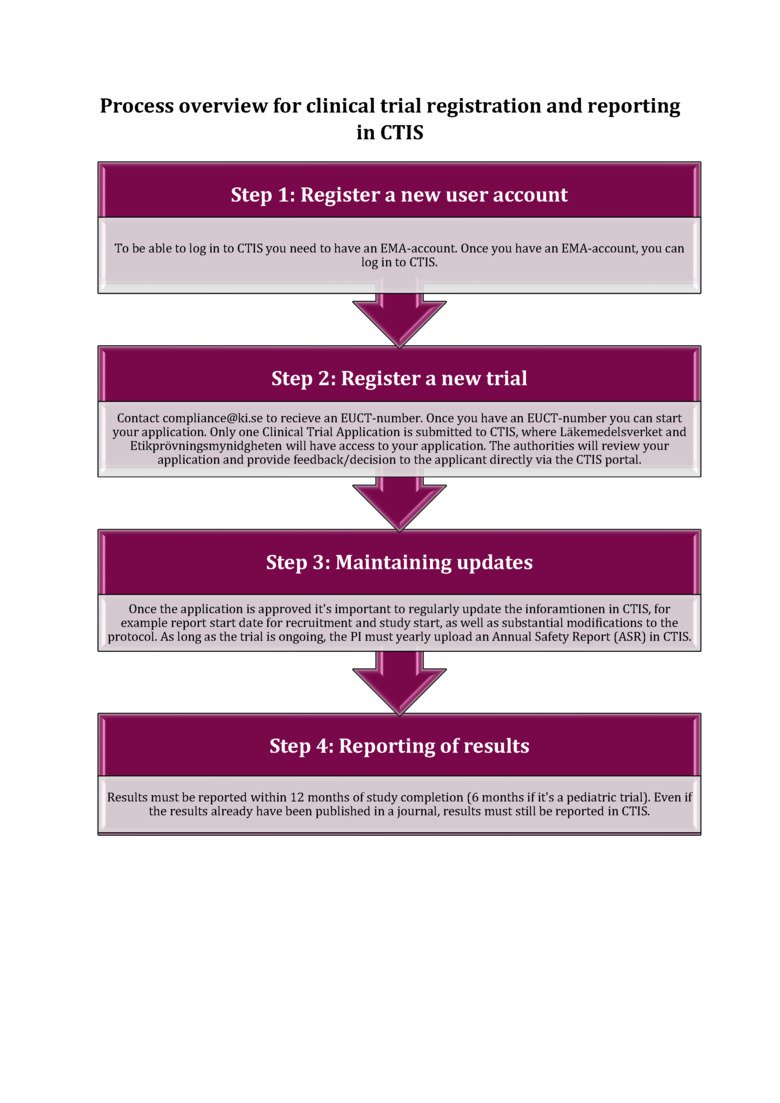
CTIS
Below you will find a flow chart with a brief description of the different steps of registering a clinical trial in CTIS. For more detailed information on each step, please visit our webpage on CTIS.