Clinical Research
Clinical research encompasses experiments or observations designed to improve human health.
As researchers are responsible for clinical research participant well-being and personal data, here you can access information about the regulations and procedures for clinical trials and research studies.
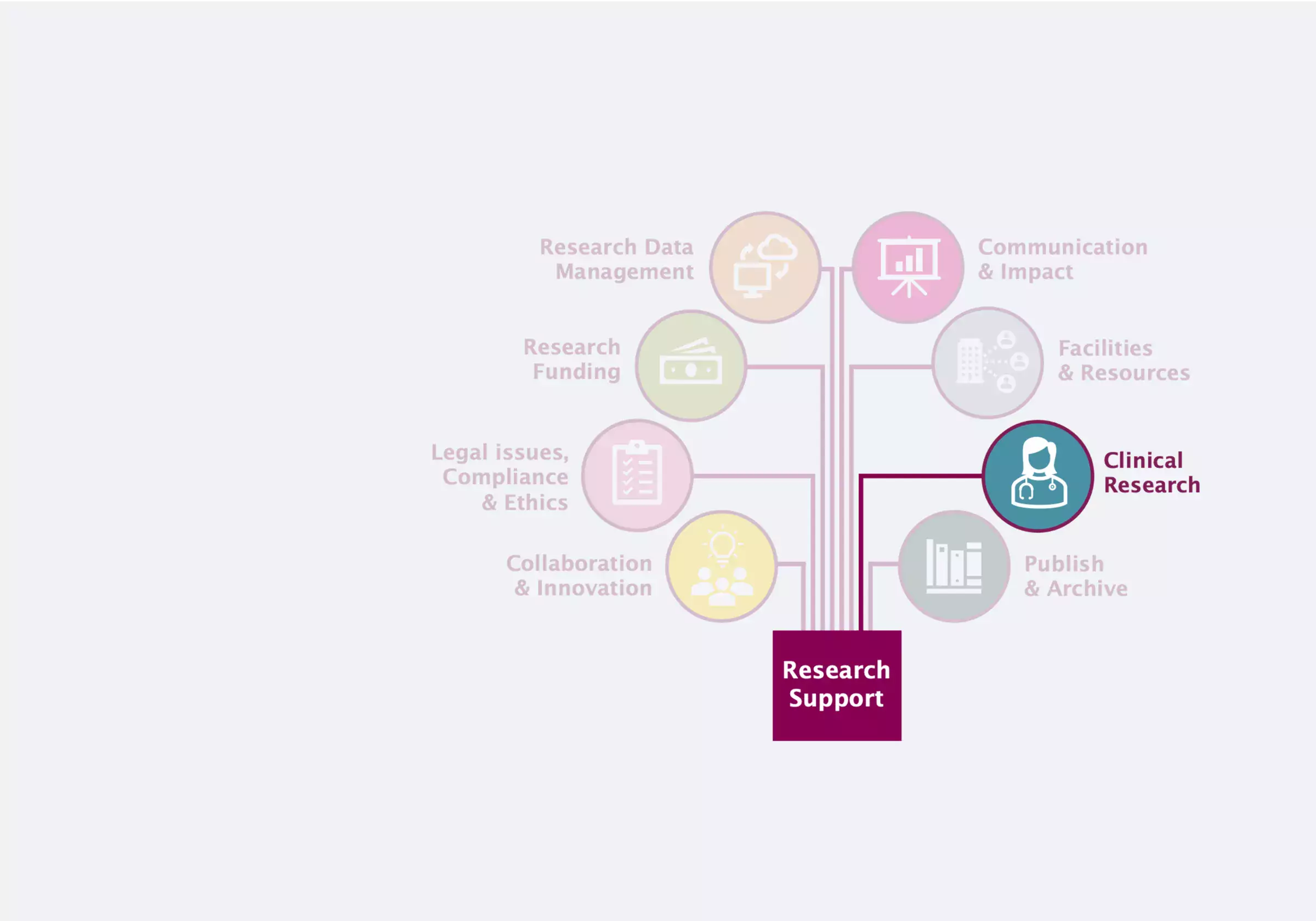 Photo: Kseniya Hartvigsson
Photo: Kseniya HartvigssonServices & support at KI
 Photo: GettyImages
Photo: GettyImages Photo: BioCalendar
Photo: BioCalendarClinical trial registration and reporting
• Regulations and requirements for clinical trials and studies
• Process of registration and reporting
• Clinical trial transparency at KI
• FAQ & Support
 Photo: GettyImages
Photo: GettyImages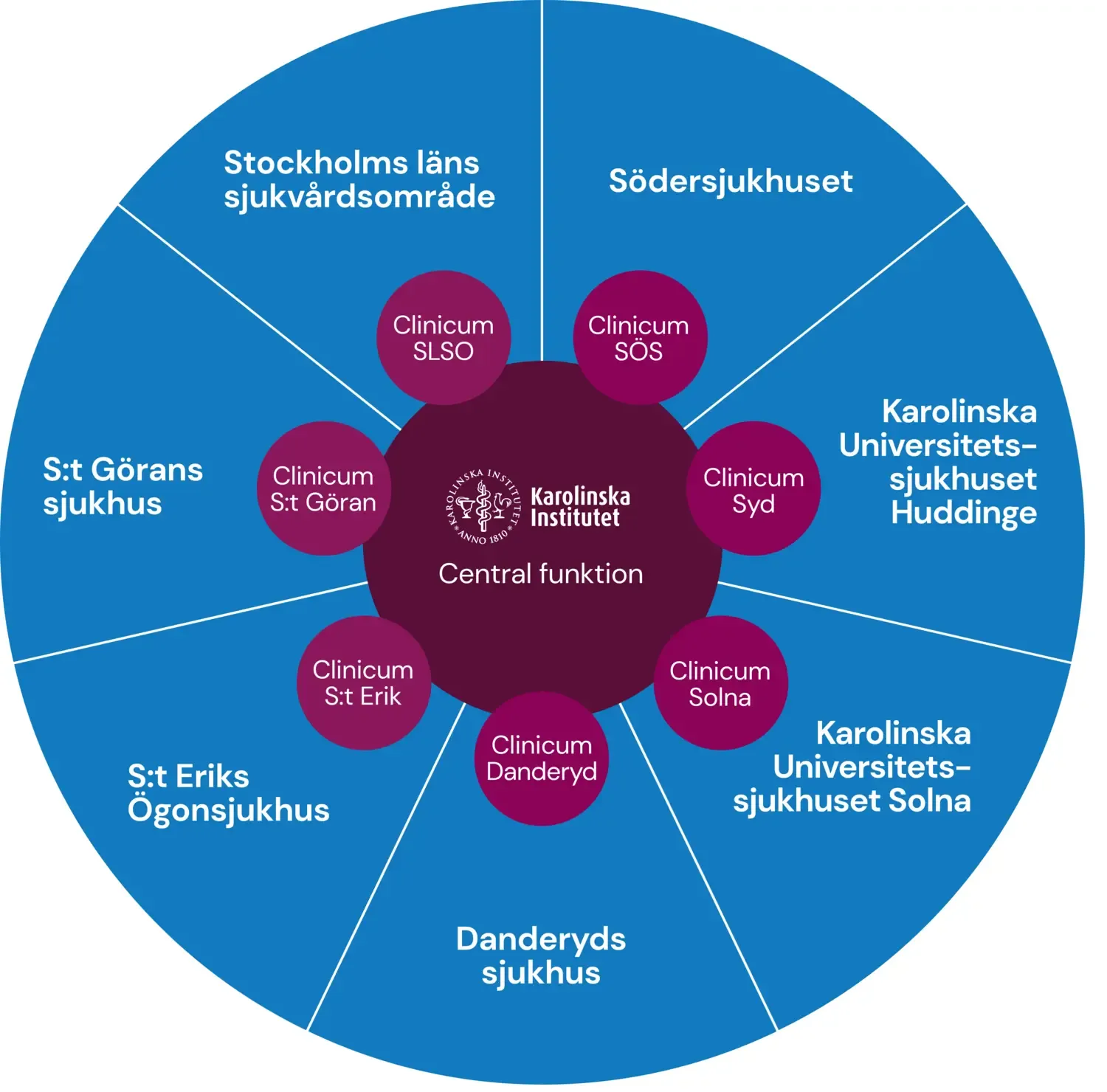 Photo: N/A
Photo: N/AClinicum: methodological support for clinical and translational research
Clinicum offers free scientific advice and methodological support and guidance in the planning and execution of research projects. Clinicum is aimed at researchers who are employed or affiliated with KI or Region Stockholm.
External resources
 Photo: GettyImages.
Photo: GettyImages. Photo: Karolinska universitestssjukhuset
Photo: Karolinska universitestssjukhusetKarolinska Trial Alliance (KTA)
KTA is a specialised, regulatory unit that provides services and courses in the planning, implementation and completion of clinical studies at cost price. We are part of Karolinska University Hospital with the task of supporting both academia and industry within the Stockholm-Gotland Region.
 Photo: Pixabay CC0
Photo: Pixabay CC0Clinical studies in Sweden: step-by-step
What does the study process look like and what needs to be considered when conducting a clinical study?
This webpage is primarily aimed at persons working within healthcare and research.
 Photo: Getty Images
Photo: Getty ImagesClinical trials during the COVID-19 pandemic
There are challenges in performing clinical trials during the coronavirus pandemic (COVID-19) and some situations may need to be handled as Urgent Safety Measures.
