Legal issues, Compliance & Ethics within research
Funders and collaborators usually require a contract for a research project, which defines respective rights, obligations, timelines and budget. Furthermore, an ethical application must be submitted and approved before you conduct studies with research subjects, human tissue or laboratory animals. You must also protect information that is linked directly or indirectly to living persons.
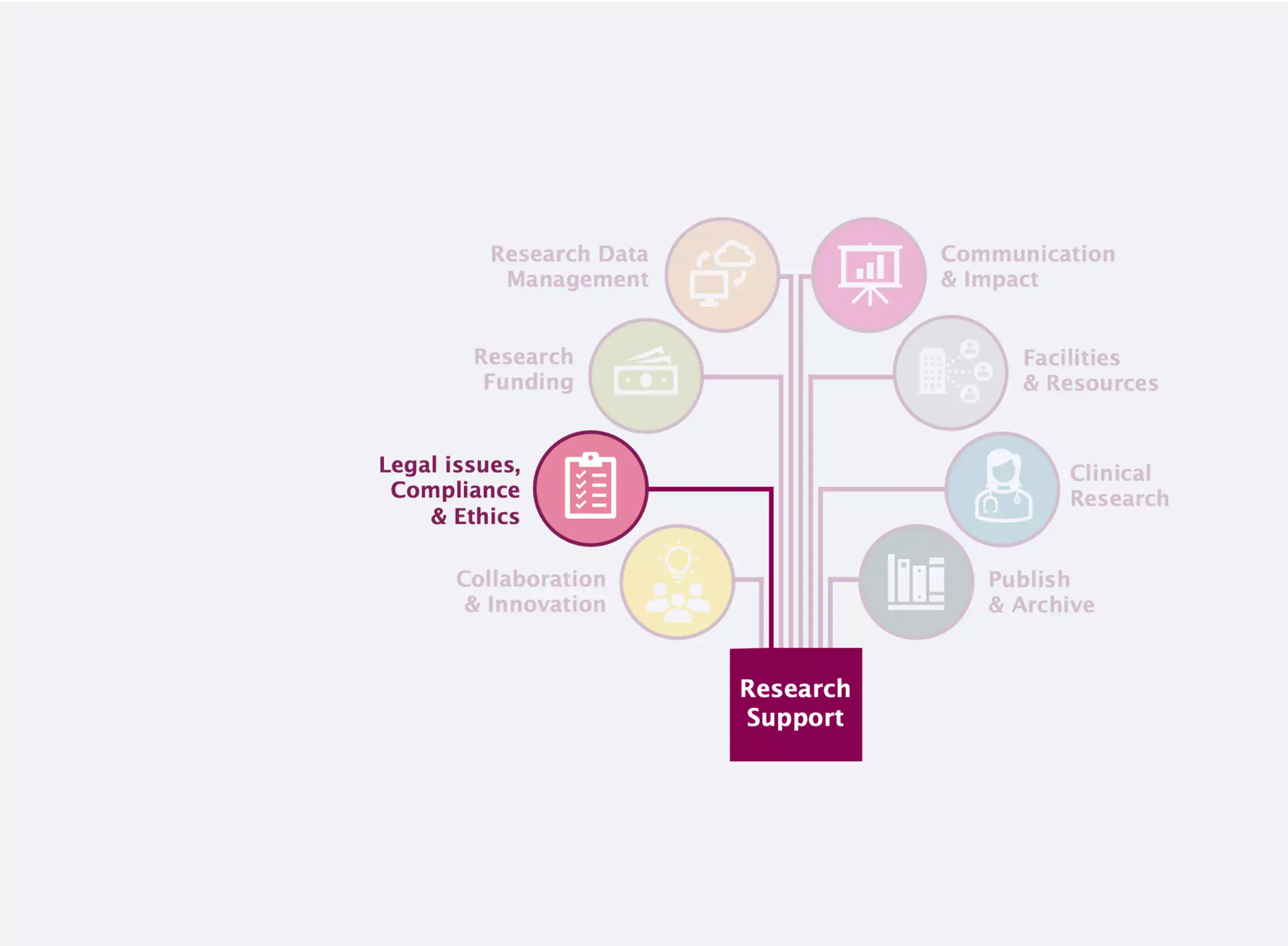 Photo: Kseniya Hartvigsson
Photo: Kseniya HartvigssonSubject
 Photo: GettyImages.
Photo: GettyImages.Research Contracts & Agreements
It is important to define roles and responsibilities with a legal agreement that covers research activities between KI and third parties: external funders, collaborators and service providers.
Here you can find information about:
• Why do you need a research contract
• Types of research contracts
• The contract process in 4 steps
• Available support at KI
 Photo: GettyImages
Photo: GettyImagesEthical issues
Select an option
Ethics at KI Ethics and compliance: Clinical studies Human ethics application Guide to the application for ethics review Choosing the responsible research principal for the ethical application Requirements for KI to be listed as research principal Ethical requirements by external research funders Ethical application for laboratory animals Compliance, ethics and open access Ethical Guidelines for International Collaboration Guidelines for research Committee for Ethics and Good Research Practice KI’s scientific representative Resources for responsible internationalisation Photo: GettyImages
Photo: GettyImagesPersonal data, security & open access
Select an option
Personal data in research (GDPR) Information protection and security Open access: policies and demands Data requirements of funders and publishers Laws and guidelines regulating research documentation and data management Process for drawing up agreements for the transfer of personal data EU ethics and data protection decision-tree Data protection impact assessments Laboratory safety Photo: Gabriel Holmbom Eg Design/CONCORD Sverige
Photo: Gabriel Holmbom Eg Design/CONCORD Sverige