Support for research contracts
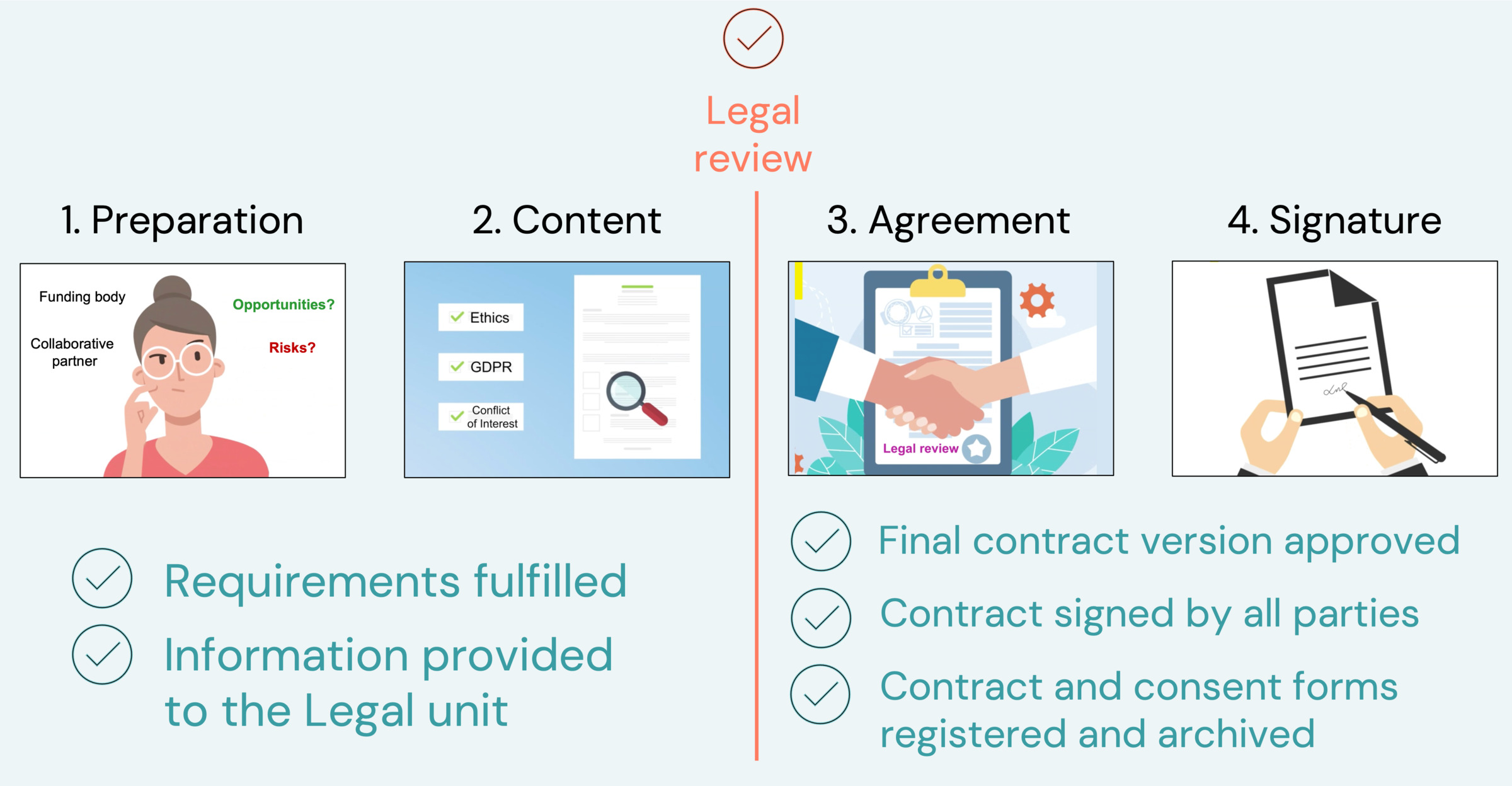
The contract process includes 4 steps. You need to provide specific information to the Legal unit before they can review your contract - see the checklist below. The Principal Investigator (PI) is usually responsible for coordinating the process and can get support from different units.
The contract process
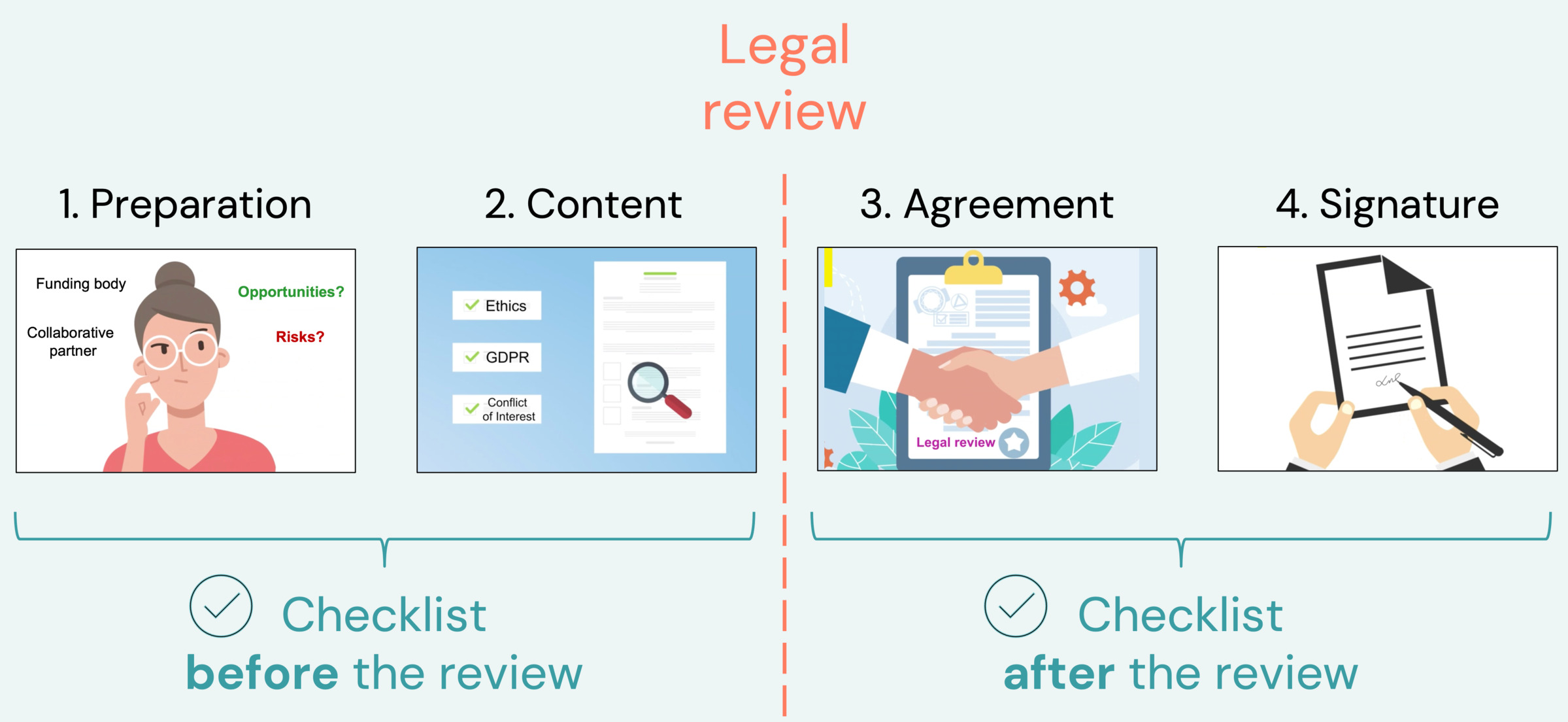
The Legal unit's checklist is also available on a separate web page: in English or Swedish
Legal review - checklist & guidelines
The Legal unit will review the contract when the requirements below are met. You should provide all necessary information when you contact the Legal unit. Scroll down the page to find additional information about each step.
During step 1. Preparation:
- Appoint a Contract Administrator - a person responsible for the process (often the PI)
- Get confirmation from Head of Department or Head of Administration
- Ensure that clinical research studies are approved by the clinic where they are going to be carried out
- Define the respective roles and responsibilities of KI and the clinic, read Checklist for clinical research projects
During step 2. Content:
- Define the contract type
- Have an appropriate/realistic budget for the contract type
- Decide the duration of the contract: start & end dates
- Prepare a detailed research plan
- Define ownership of results and the parties’ right to use the results
- Decide who at KI will participate in the project and inform the counterparty of any affiliated participants
- Decide how the results will be published - individually, by one of the parties or jointly by all parties
- Find out if prior approval for the transfer of material or data is required, read Export control of dual-use items
- Also identify:
- If ethical or animal permits are required
- If insurance is needed
- When the project involves processing of personal data (GDPR)
- Where biobank materials are handled (KI Biobank, SMB or other). MTA should also be established.
During step 3. Agreement
- Make sure that the final version is approved by the relevant official signatories. For example: Head of Research Support Office, University Director, President or Academic Vice-president.
During step 4: Signature
- Check who should sign the contract and how (for example digitally)
- Sign and send the researcher consent form to all involved KI researchers
- Register (Dnr) and archive the fully signed contract and the researcher consent forms
Scroll down to find additional information about each step.
KI guidelines in English:
Guidelines for research-related contracts (pdf)
Rules for handling secondary occupations (pdf)
Guidelines on conflict of interest (COI) (pdf)
Rules for financial conflicts of interest in research projects funded by the American federal governmental authorities (pdf)
Delegation rules for KI
Guidelines for digital signatures (pdf)
Research funding forms and KI registrations
KI guidelines in Swedish:
Riktlinjer för forskningsrelaterade avtal (pdf)
Regler för hantering av bisysslor (pdf)
Riktlinjer om jäv (pdf)
Anvisningar för hantering av ekonomiska intressekonflikter i forskningsprojekt finansierade av amerikanska federala hälsomyndigheter (pdf)
Delegationsordning vid KI
Riktlinjer för elektroniska underskrifter (pdf)
Additional information & support
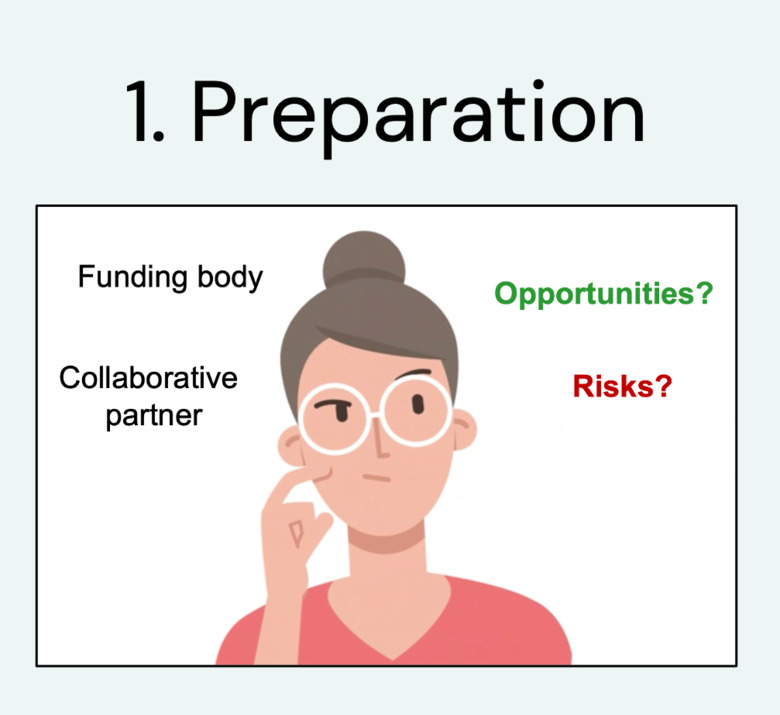
During step 1. Preparation
You need to identify a collaborative partner/funding body and assess opportunities/risks.
Then you need to appoint a person who will be responsible for coordinating the processa and get approval from your department.
I want to:
Each research contract requires a person to be responsible for coordinating the process - often the Principal Investigator (PI) or in some cases a specifically appointed person at the department. Always contact your department administration to identify a responsible person and other involved researchers (affiliated, consultants, students, PhD students).
The Contract Administrator (often the PI) can throughout the process get support from different units in order to:
- Organise internal communication with the legal unit and other support functions
- Send the agreement draft to all parties and to avtal@ki.se and engage in all revisions
- Negotiate with the counterparty
- And must know the content of:
Always discuss the collaboration plans with your Head of Department and/or Head of Administration:
- They should review the contract and decide if it also needs to be reviewed by the Legal unit to provide recommendations
- They approve the contract based on the Legal unit's recommendations
- If necessary, they can also contact the External Engagement Office to get additional support in the preparation process or the Compliance and Data Office for ethical permit and data handling issues
Get confirmation from Head of Department or Head of Administration that:
- The proposed scientific and financial terms of the contract are acceptable
- The contract is appropriate for the activities, guidelines and ethical principles of KI
- KI is the contractual party and not a researcher through a secondary occupation, read the Rules for handling secondary occupations
- The counterparty is suitable. An assessment of the risks associated with the collaborator should be made, for example to assess if KI’s reputation can be damaged by being associated with the collaborator or its operations.
- A responsible Contract Administrator is appointed (often the PI)
External Engagement Office creates new opportunities for KI researchers for partnerships with industry and other external organisations, and provides support in setting up and managing long-term structured partnerships.
EEO can help you to:
- Identify type of collaboration and appropriate agreement
- Understand the difference between commissioned and collaborative research
- Get advice and support for industry collaborations
- Discuss risks in choice of collaborative partner:
- Is the collaborator appropriate?
- Some companies might have weak economies
Grants Office provides support for KI internal research funding and pre-award support for external funding. Contact grantsoffice@ki.se
GO can help you to:
- Apply for international grants
- Review the grant funding terms. If funding from several grants is used within a project you need to ensure the terms of the different funding agencies are compatible.
- Negotiate EU grant agreements
- Discuss risks in choice of financing organisation
If the agreement is in the form of commissioned research, you will need to assure there is a full cost coverage in the project budget, including all salary costs, overhead and indirect INDI-costs
Compliance & Data Office provides researchers with support and advice on research documentation, data and information management, Electronic Logbook (ELN), laws and regulations for externally funded projects, "non-financial compliance", as well as clinical trials, ethics and personal data management.
Useful links:
You need to be aware of any possible situations of conflict of interest. A general principle is that KI will not have contracts with companies in which the KI employees have significant responsibility or influence.
We recommend you to contact HR office. Their Negotiation Unit (förhandlingsenheten) is responsible for KI:s negotiating activities and local collective agreements. The unit provides advice and support for general questions regarding personnel matters and labor law.
Also read:

During step 2. Content
You need to understand what type of agreement is important in your case and what should be included.
Before the Legal unit can review your research agreement draft, you should identify all financial and regulatory issues to make the review process as efficient as possible.
I want to:
Purpose: A research contract is often required to define respective expectations, rights, obligations, ethical issues, financial terms, timelines and other formal aspects. Such agreements also help to reduce the risk of costly disputes, to prevent possible problems and conflicts that the parties may have after the agreement has been signed.
Content: The formulation of contracts must always be customised to the situation, and must include guidelines for governing the rights and utilisation (present) and possible further development of the result (future).
Read more: Research contract: purpose, content and different types
There are many different types of contracts, depending on the nature of the partnership, for example:
- Confidentiality agreement
- Commissioned research agreement
- Research Collaboration Agreement
- Material Transfer Agreement
- Consortium Agreement
- Data transfer agreement
- Purchase of goods or services, contact the Purchase and public procurement unit (inköp och upphandling)
- Donation/sponsorship, read the Rules on donations and sponsorships, contact Development Office do@ki.se
- Grant agreement, contact grantsoffice@ki.se for international grants
Also read:
Support: contact the Research Data Office rdo@ki.se or the External Engagement Office
You need to contact your department’s economy administrator to define the following:
- Financial contributor
- Client, full cost coverage is required for commissioned research
- A suitable payment plan
If the agreement is in the form of commissioned research, you will need to assure there is a full cost coverage in the project budget, including all salary costs, overhead and indirect INDI-costs
You need to describe the responsibilities and tasks of each party (e.g. project plan, milestones).
You should also list KI background, see Guide till Vinnovas villkor om nyttjanderätt
You can read:
Research projects often require ethical permits. Projects may also include transfer of data and material between collaborators - a Data Transfer Agreement is then required if personal information is used during the project.
Identify if ethical or animal permits are required
- Read: Ethical issues at KI
- You need to identify if KI must be the principal research party
- Contact Compliance & Data Office for support
Identify if insurance is required
- Insurance for research subjects
- Insurance terms at KI
- Insurance for international students
- Contact Compliance & Data Office or HR Office for support
Identify where the project involves the processing of personal data (GDPR)
- Prepare a Data Management Plan
- Carry out a data protection impact assessment when necessary according to these Instructions
- Define the roles/responsibilities of KI and other parties
- Register the personal data processing activities at KI (registeranmälan) with KI Data Protection Officer
- Contact Research Data Office or KI Data Protection Officer for support
Identify when biobank materials are handled - if you collect human biological samples
KI Biobank for researchers:
- Helps to establish the necessary agreements
- Offers sample handling
- Provides robust IT systems ensuring full traceability
- For questions contact the biobank coordinator at biobank@ki.se or call 08-524 820 03
Stockholms Medicinska Biobank (SMB) - human samples from healthcare
The data management process is divided into 5 steps.
Find rules and guidelines at the webpage KI:s environmental and sustainability management system.
You can also contact environmental coordinators: miljo@ki.se
If funding from several grants is used within a project, be sure the terms of the different funding agencies are compatible.
- Follow the procurement process for the purchase of goods and services. We recommend you to contact the Property and Facilities Office (inköp- och upphandling)
- If the agreement is in the form of commissioned research, you will need to assure there is a full cost coverage in the project budget, including all salary costs, overhead and indirect INDI-costs

During step 3. Agreement
You need to draft, negotiate and finalise an agreement that is legally correct - e.g. reviewed by the Legal unit.
Make sure that the final version is approved by the relevant official signatories.
I want to:
We recommend to use KI templates to make the review process as efficient as possible.
- Send an e-mail to avtal@ki.se to receive agreement templates
- You will get an automatic reply with the case number - always reply to this e-mail
- In the automatic reply you will find a list of questions - please provide correct information so that the Legal unit can define what type of agreement is needed in your case
- Don't send questions regarding the same case in a new e-mail, otherwise you will receive a new case number
The final version is approved by the relevant official signatories (for example, Head of Research Support Office, University Director, President or Academic Vice-president).
The Head of the Research Support and External Relations Office together with the Head of Department makes decisions for:
- Funding agreements and applications to the EU, the National Institutes of Health (NIH) and other foreign donors
- Cooperation agreements with other grant recipients, possible additions and changes to existing applications and agreements
The Legal unit will review the contract only when the requirements are met and all necessary information is provided to the Legal unit.
Head of Department and/or Head of Administration should review the contract and decide if it also needs to be reviewed by the Legal unit. The Head of Department then approves the contract based on the Legal unit's recommendations.
The Contract Administrator (often the PI) can throughout the process get support from different units in order to:
- Organise internal communication with the legal unit and other support functions
- Send the agreement draft to all parties and to avtal@ki.se and engage in all revisions
- Negotiate with the counterparty
The Legal unit at KI:
- Offers research agreement reviewing and negotiations only for central agreements that don't have a PI
- Can provide advice on the terms and conditions of collaborations, and assistance with the review and interpretation of contracts
- Has contract templates, forms and additional documents that might be needed for project planning and contract drafting
- Provides recommendations when advising on and reviewing contracts
- Does not represent KI in contract negotiations other than in exceptional cases and when agreed by the legal unit

During step 4. Signature
When the agreement's final version has been reviewed by the Legal unit, the agreement must be signed by all parties & other stakeholders, before the research project starts.
When the agreement's final version has been reviewed by the Legal unit, the agreement must be signed before the research project starts. The agreement can't only be signed by the academics themselves and requires signatures from all parties and in some cases from other stakeholders.
Who should sign the agreement?
According to the Delegation rules for KI:
- Head of the Department and in some cases the Head of Administration
- Principal Investigator (PI)
- International funding agencies also require the signature of the Research Support and External Relations Office together with in the Head of Department
In some cases, the University Director, Academic Vice President or President also should sign the agreement together with the Head of the Department.
- Agreements can be signed digitally according to KI’s decision making procedures and Delegation Rules
- People signing the agreement should do it in the correct order
- Everyone should use the same type of e-signature service
- A combination of e-signature and manual signatures in the same document is not possible
- Indicate a date in the contract, the international standard recommends YYYY-MM-DD
- Head of Administration can guide you through the signature process
If you collect manual signatures (not digital), remember to print a few copies of the agreement so that there is a separate copy for signing by all parties.
Read the Guidelines for digital signatures
All researchers involved in the project and described in the contract must sign a Researcher consent form, assuring they have read and understood the terms and conditions of the agreement and that they will comply with them.
During signature of the contract you need to send the researcher consent form to all KI researchers involved in the project to get their signature.
Both the fully signed contract and the researcher consent form should be registered and archived by your department.
If you have questions about registration of documents, contact registrator@ki.se
Or visit the web pages:
Summary: the contract process