Plan your research data management
Here you will find information on why research has to be documented, what needs to be documented and what to think about before you start to create, collect and store your research data.
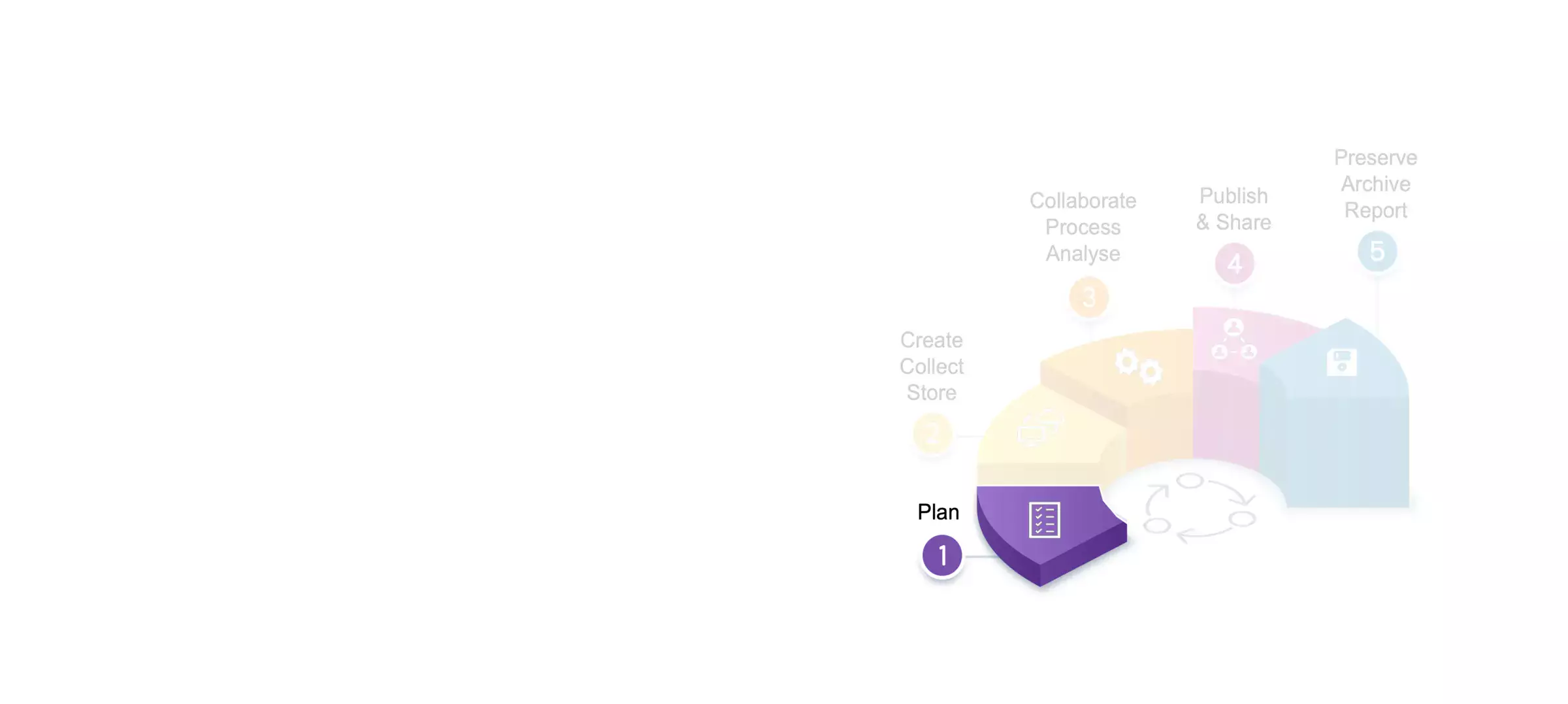 Photo: Kseniya Hartvigsson
Photo: Kseniya HartvigssonResearch Data Management in 5 steps:
 Photo: Pixabay CC0
Photo: Pixabay CC0Why should research be documented
Research documentation at KI should be done in such a way that it is possible for co-workers and external peers to follow and review the research.
 Photo: Thinkstock
Photo: ThinkstockData Management Plans (DMP)
Quality assured and correct data management is often facilitated by a well-thought-out data management plan (DMP)
What and how to document
 Photo: Marie Dahlberg
Photo: Marie DahlbergWhat should be documented
Research documentation should cover the intellectual and practical sides of research, and the administrative documents pertaining to the research.
 Photo: Okänd
Photo: OkändElectronic documentation
KI ELN is an electronic notebook (lab/log-book) that is the platform of choice for electronic research documentation at KI.
Data security and personal data
 Photo: Thinkstock
Photo: ThinkstockData protection and data security
Information security is largely about ensuring that the right information is available to the right person at the right time.
 Photo: RDO
Photo: RDOPersonal data in research
The General Data Protection Regulation for the processing of personal data regulates research data management.
 Photo: Getty Images
Photo: Getty ImagesResearch principal & responsibility for personal data
For clinical projects, make sure you know what contracts/agreements are necessary, who the research principal is and how the responsibility for personal data is set up for the project
The FAIR principles
 Photo: No one
Photo: No oneThe FAIR-principles
The EU and Horizon 2020 state that research data must meet the so-called FAIR principles. Also major initiatives such as the European Open Science Cloud (EOSC) connect to FAIR.
The concept FAIR stands for data to be:
Findable Accessible Interoperable ReusableEthics
 Photo: Pixabay CC0
Photo: Pixabay CC0Documentation for the human ethics application
Are you going to perform studies with research subjects, human tissue or sensitive personal information? If so, an ethics application is required
 Photo: iStock
Photo: iStockGuide to the application for ethics review
KI has drafted a ”Guide to the application for ethics review” which is intended to be a support for researchers when they are going to fill in their ethics application.
 Photo: GO
Photo: GOEthical requirements for external research funders
Ethical requirements for external research funders
 Photo: RDO
Photo: RDO
